How Many Atoms Are In Vitamin C
 | If you already know what ATOMS and ELEMENTS and MOLECULES are and want to dive straight into polymers, click here. |
OK, so -- what's an atom and what does it have to do with molecules?
Let's talk tiny, and I mean really itty-bitty. ATOMS are the basic building blocks of everything you can see around you, and even lots of things you can't see, like the air that you breathe. Atoms are so small that there are millions and billions and trillions in the tiniest speck you can see. Solids, liquids, gases - all matter - are made up of atoms (or other things, like molecules, that are made from atoms)!
 ELEMENTS are the kinds of atoms that we can have. Carbon is an element, hydrogen is an element, and so is oxygen. (We can call them by their names, or by their symbols - C for carbon, H for hydrogen, and O for oxygen.) All the elements are listed out in a periodic table. If it's in the table, it's an element!
ELEMENTS are the kinds of atoms that we can have. Carbon is an element, hydrogen is an element, and so is oxygen. (We can call them by their names, or by their symbols - C for carbon, H for hydrogen, and O for oxygen.) All the elements are listed out in a periodic table. If it's in the table, it's an element!
Atoms can join together - they form bonds together - to make MOLECULES. For example, two atoms of hydrogen hook together to form a molecule of hydrogen, H2 for short. Pretty simple, huh?
For example, two atoms of hydrogen hook together to form a molecule of hydrogen, H2 for short. Pretty simple, huh?
Click here to take a look at a 3-D model of hydrogen (H2).
Now, if you stick an oxygen atom in between those two hydrogens, you have a molecule of water - H2O!
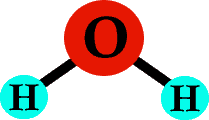
Good old H2O!
Click here to see water (H2O) in 3-D.
Each type of atom typically forms the same number of bonds (they tend to be stubborn that way). For example, a hydrogen atom forms one bond, an oxygen atom forms two, and carbon forms four bonds. Look at that molecule of water again - each hydrogen has one bond, and the oxygen in the middle has two bonds.
Molecules can be much bigger. One molecule of vitamin C is made up of 20 atoms (6 carbons, 8 hydrogens, and 6 oxygens - that's C6H8O6). If you take those 20 atoms of vitamin C and mix them around, bonding them together in a different order, you'll have a totally different molecule that not only looks different, it acts different. (If your legs were sticking out of your head, you'd act weird too!!)
So, molecules are atoms stuck together, but not just any old way. Changing which atom is bonded to which can change the properties of a molecule, that is, how it looks and acts - and that changes how a whole BUNCH of molecules hanging out together will look and act. For example, water is a liquid, hydrogen is a gas, and vitamin C is a solid.
"SO WHAT?!!" you ask. Well, when we talk about polymers, how the atoms are bonded to each other can have a HUGE impact on what something made out of those polymers feels like and reacts when you bash it or step on it or throw it against the wall. Like, will it stick to the wall or bounce off?
A Bonding Experience
The kind of bond we've been talking about here is called a "covalent bond," meaning that it's made by sharing electrons between two atoms.
More about bonding and the structure of atoms here, otherwise ....
Let's talk POLYMERS !!
How Many Atoms Are In Vitamin C
Source: https://www.pslc.ws/macrog/kidsmac/atoms.htm

0 Komentar